
Your Gift Can Make 2x the Impact
Your Gift Can Make 2x the Impact
A gift can go twice as far during our February $100,000 2x Match. Your contribution by Feb. 27 will fuel Alzheimer’s research and help provide essential care and support.
Donate NowCMS Treatment Coverage Policy: What It Means for People Living with Alzheimer’s
In April 2022, the Centers for Medicare & Medicaid Services (CMS) issued an unprecedented decision restricting Medicare coverage of Food and Drug Administration (FDA)-approved treatments that target amyloid for Alzheimer's disease. But following extensive efforts by the Alzheimer's Association, bipartisan members of Congress, state attorneys general, clinicians and, most importantly, advocates from communities all across the country, CMS has updated its coverage plan for traditionally approved FDA treatments.
Medicare now will cover FDA-approved monoclonal antibody treatments that receive traditional approval if clinicians participate and enter data in a simple registry. This policy applies to all currently FDA-approved monoclonal antibody treatments, including lecanemab (Leqembi®) and donanemab (Kisunla™), and others that may receive approval.
In addition, in October 2023, CMS issued a new policy that expands coverage of brain amyloid positron emission tomography (PET) imaging for the diagnosis of Alzheimer's disease. Access to amyloid PET imaging for Alzheimer's diagnosis is essential in the new era of treatments.
Your voice is important to the fight
By generously sharing your time and resources, you have brought us to this pivotal moment — but we must keep going. Learn about all of the ways people like you are moving the needle for the cause.
More information
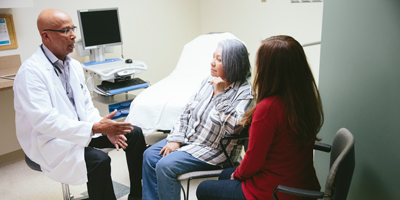
Navigating Treatment Options

Governance and Financial Transparency

Mild Cognitive Impairment (MCI)
We're here for you
The Alzheimer’s Association 24/7 Helpline (800.272.3900) is available around the clock, 365 days a year. Through this free service, specialists and master’s-level clinicians offer confidential support and information to people living with dementia, caregivers, families and the public.
About the Alzheimer’s Association
The Alzheimer's Association leads the way to end Alzheimer's and all other dementia — by accelerating global research, driving risk reduction and early detection, and maximizing quality care and support. Our vision is a world without Alzheimer's and all other dementia®.
