Earlier Diagnosis
What if we could diagnose Alzheimer's disease before symptoms started? The hope is, future treatments could then target the disease in its earliest stages, before irreversible brain damage or mental decline has occurred. Research on new strategies for earlier diagnosis is among the most active areas in Alzheimer's science, and funding from the Alzheimer's Association has spurred significant advances and steady progress.
Biomarkers for earlier detection
Current diagnosis of Alzheimer's disease relies largely on documenting mental decline, at which point, Alzheimer's has already caused severe brain damage. Researchers hope to discover an easy and accurate way to detect Alzheimer's before these devastating symptoms begin.
Experts believe that biomarkers (short for "biological markers") offer one of the most promising paths. A biomarker is something that can be measured to accurately and reliably indicate the presence of disease, such as fasting blood glucose (blood sugar) level, which indicates the presence of diabetes if it is 126 mg/dL or higher.
 Several potential biomarkers are being studied for their ability to indicate early stages of Alzheimer's disease. Examples being studied include beta-amyloid and tau levels in cerebrospinal fluid (CSF) and brain changes detectable by imaging. Recent research suggests that these indicators may change at different stages of the disease process.
Several potential biomarkers are being studied for their ability to indicate early stages of Alzheimer's disease. Examples being studied include beta-amyloid and tau levels in cerebrospinal fluid (CSF) and brain changes detectable by imaging. Recent research suggests that these indicators may change at different stages of the disease process.
Before a biomarker can be used in medical clinics, it must be validated, in which multiple studies in large and diverse groups of people establish that it accurately and reliably indicates the presence of disease. Furthermore, the laboratory methods used to measure the biomarker must be shown to be stable and reliable.
Currently, there are some FDA-approved tools that, when applicable, can be used to aid in diagnosis of people with symptoms of Alzheimer’s or another dementia (e.g., brain imaging). Some of these tools have a wealth of research and clinical data to support their use in the clinic (e.g., biomarkers in CSF), while other emerging biomarkers are promising but still under investigation (e.g., blood tests and genetic risk profiling).
Brain imaging/neuroimaging
Neuroimaging is regularly used today for early detection of Alzheimer’s. Research continues to evolve on promising new and advanced brain imaging techniques.
Imaging technologies used in Alzheimer's research
- Structural imaging provides information about the shape, position or volume of brain tissue. Structural techniques include magnetic resonance imaging (MRI) and computed tomography (CT).
- Functional imaging reveals how well cells in various brain regions are working by showing how actively the cells use sugar or oxygen. Functional techniques include positron emission tomography (PET) and functional MRI (fMRI).
- Molecular imaging uses highly targeted radiotracers to detect cellular or chemical changes linked to specific diseases. Molecular imaging technologies include PET and fMRI.
Structural imaging
Having shown that the brains of people with Alzheimer's shrink significantly as the disease progresses, structural imaging research also has shown that shrinkage in specific brain regions such as the hippocampus may be an early sign of Alzheimer's. Scientists now have some agreement on standardized values for brain volume loss (sometimes measured over time) that would indicate the presence of disease or disease progression.
Today, a standard workup for Alzheimer's disease may include structural imaging, and these tests are currently used to define pre-existing tissue damage associated with neurodegeneration and to rule out other conditions that may cause symptoms similar to Alzheimer's but require different treatment. Structural imaging such as MRI can reveal tumors, evidence of small or large strokes, damage from severe head trauma, or a buildup of fluid in the brain, as well as detect underlying conditions that may preclude an individual from certain treatments.
Functional imaging
Functional imaging research suggests that those with Alzheimer's typically have reduced brain cell activity in certain regions. For example, studies with fluorodeoxyglucose (FDG)-positron emission tomography (PET) indicate that Alzheimer's is often associated with reduced use of glucose (sugar) in brain areas important in memory, learning and problem-solving. According to Medicare recommendations, an FDG-PET scan is considered a reasonable test for people with a recent diagnosis of dementia and documented cognitive decline of at least six months who meet diagnostic criteria for both Alzheimer’s and frontotemporal dementia.
Molecular imaging
Molecular imaging, which also uses PET scans, is among the most active areas of research aimed at finding new approaches to diagnose Alzheimer's in its earliest stages. Molecular strategies may detect biological clues indicating Alzheimer's is under way before the disease changes the brain's structure or function, or takes an irreversible toll on memory, thinking and reasoning. Molecular imaging also may offer a new strategy to monitor disease progression and assess the effectiveness of next-generation, disease-modifying treatments. Several molecular imaging compounds are being studied, and four have been approved for clinical use:
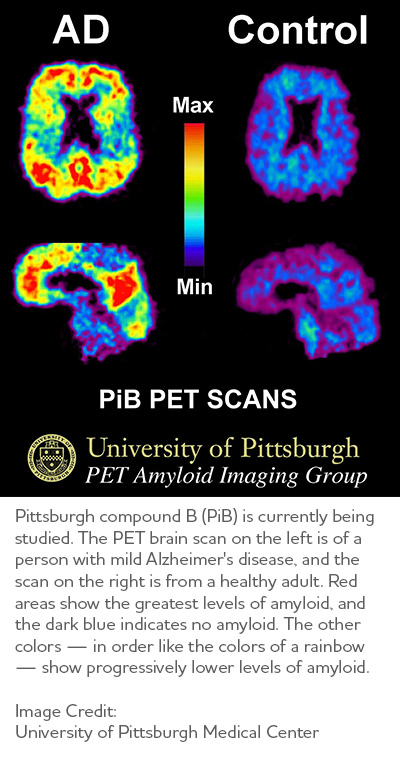
- Florbetaben (Neuraceq®), Florbetapir (Amyvid®) and Flutemetamol (Vizamyl®) have been approved for detection of beta-amyloid in the brain.
- Flortaucipir F18 (Tauvid®) has been approved for detection of tau in the brain.
Even though amyloid plaques in the brain are a characteristic feature of Alzheimer's disease, their presence alone cannot be used to diagnose the disease. Today, a diagnosis of Alzheimer’s is based on the evaluation of several things, including the presence of amyloid plaques. Your doctor may perform tests to evaluate your memory, order laboratory tests or perform a molecular imaging test (e.g., PET scan) to confirm an Alzheimer’s diagnosis or rule out other diseases that may cause similar symptoms.
Cerebrospinal fluid (CSF) tests
CSF is a clear fluid that bathes and cushions the brain and spinal cord. Adults have about 1 pint of CSF, which physicians can sample through a minimally invasive procedure called a lumbar puncture, or spinal tap. Research suggests that Alzheimer's disease in early stages may cause changes in CSF levels of multiple markers such as tau and beta-amyloid, two markers that form abnormal brain deposits strongly linked to Alzheimer's. Another potential marker is neurofilament light (NfL), an increased level of which has been found in neurodegenerative diseases such as Alzheimer’s.
One challenge researchers face is that analysis of biomarker levels in the same sample can often vary significantly from institution to institution and across different testing platforms. Great strides have been made in standardizing the measurement of these markers in research and clinical care. CSF tests are currently used by dementia specialists to aid in the diagnosis of Alzheimer's, and research continues to develop and standardize new markers that will aid in diagnosis and detection of other dementias.
One CSF Amyloid Ratio test, Lumipulse®, received FDA approval and is a new diagnostic tool that clinicians can use to detect amyloid in CSF, which can be predictive of amyloid changes in the brain.
The Alzheimer's Association convened a multidisciplinary workgroup and published appropriate use criteria to guide the safe and optimal use of the lumbar puncture procedure and CSF testing for Alzheimer's disease.
Blood tests
Researchers are investigating whether consistent and measurable changes in blood levels of specific markers may be reliably associated with Alzheimer’s related changes. These markers may include tau, beta-amyloid or other biomarkers the could be measured before and after symptoms appear.
An urgent need exists for simple, inexpensive, non-invasive and easily available diagnostic tools such as blood tests to diagnose the disease. These testing technologies would support drug development by helping to identify and follow treatment effectiveness in clinical trial participants and to increase the possibility of early detection, diagnosis and intervention. A blood test would also enable interpretation and understanding of the progression of Alzheimer’s in larger and more diverse populations.
Today, blood tests are already improving the design of clinical trials, and they are being used in some specialty care centers. In the future, they are very likely to revolutionize the diagnostic process for Alzheimer’s and all other dementia. The use of these tests in trials and at the doctor's office must be done in a careful and controlled way because much more research is still needed before they can be routinely used in the clinic, as researchers are still working toward creating standardized and validated tests that will deliver reliable results for all individuals.
There are a few blood tests currently on the market that can be ordered by health care providers to aid in the diagnosis of memory complaints. These tests do not yet have FDA approval. At this time, it is recommended that blood tests only be used by specialty care doctors who are seeing patients with memory complaints. They are not recommended for individuals who do not have any cognitive or memory symptoms.
The currently available tests may predict the presence of amyloid changes in the brain or the presence of neurodegenerative disease or neuronal damage. These blood tests cannot be used as a stand-alone test to diagnose Alzheimer’s disease or any other dementia; they will be used as part of a diagnostic workup with other exams.
Genetic risk profiling
23 Chromosome Pairs; 4 Alzheimer's Genes Identified:
Amyloid precursor protein (APP),
discovered in 1987, is the first gene with mutations found to cause an inherited form of Alzheimer's.
Presenilin-1 (PS-1),
identified in 1992, is the second gene with mutations found to cause inherited Alzheimer's. Variations in this gene are the most common cause of inherited Alzheimer's.
Presenilin-2 (PS-2),
discovered 1993, is the third gene with mutations found to cause inherited Alzheimer's.
Apolipoprotein E-e4 (APOE4),
discovered in 1993, is the first gene variation found to increase risk of Alzheimer's and remains the risk gene with the greatest known impact. Having this mutation, however, does not mean that a person will develop the disease.
Scientists have identified three genes with rare variations that cause Alzheimer's (Dominantly Inherited Alzheimer’s Disease) and several genes that increase risk but don't guarantee that a person will develop the disease. Investigators worldwide are working to find additional risk genes as well those that may decrease an individual’s risk. As more effective treatments are developed, genetic profiling may become a valuable risk assessment tool for wider use.
Genetic testing for APOE-e4, the strongest risk gene in some populations, is included in some clinical trials to identify participants at high risk for Alzheimer’s disease or risk side effects that may be associated with approved treatments. Learn more about genetics and Alzheimer's disease.
