About the ALZ WW-FNFP grant
The World Wide FINGERS (WW-FINGERS) Network launched in 2017 as the first global network of multidomain lifestyle intervention trials for dementia risk reduction and ultimately prevention. Studies that participate in the WW-FINGERS network aim to apply, test and optimize the FINGER-like model to reduce risk across the spectrum of cognitive decline in different geographical, cultural and economic settings in individuals at greater risk as they age.
To support the expansion and opportunity for advancing this research, the Alzheimer’s Association is launching a pilot funding program for WW-FINGERS Network projects. The ALZ WW-FNFP will provide initial/startup funding and/or opportunities for ongoing studies to initiate WW-FINGERS like studies, to expand these studies or to add/leverage unique opportunities that enhance the study. This will be a competitive funding program and all submissions will be reviewed.
This grant is open to U.S. and international researchers.
Grant RFP
View program objectives, eligibility, submission process and more.
Grant Pre-submission Inquiry
Download and complete the WW FINGERS Network Study pre-submission inquiry. Email a completed inquiry to grantsapp@alz.org. Inquiries will be reviewed by the Alzheimer’s Association. Those who meet the program objectives will be invited to submit full applications.
Program objective
The World Wide FINGERS (WW-FINGERS) Network launched in 2017 as the first global network of multidomain lifestyle intervention trials for dementia risk reduction and ultimately prevention. Studies that participate in the WW-FINGERS network aim to apply, test and optimize the FINGER-like model to reduce risk across the spectrum of cognitive decline in different geographical, cultural and economic settings in individuals at greater risk as they age. In order to succeed in providing broad accessibility to and representation of the global community, there is a significant need for seed funding and opportunities to advance a WW- FINGERS network study. The WW-FINGERS network is a collaboration led by the scientific lead, Dr. Miia Kivipelto and the FINGER team and the Alzheimer’s Association. To support the expansion and opportunity for advancing this research, the Alzheimer’s Association is launching a pilot funding program for WW-FINGERS Network projects. The Alzheimer’s Association WW- FINGERS network funding program (ALZ WW-FNFP) will provide initial/startup funding and/or opportunities for ongoing studies to initiate WW-FINGERS like studies, to expand these studies or to add/ leverage unique opportunities that enhance the study. This will be a competitive funding program and all submissions will be reviewed.
Program overview
The WW-FINGERS Network includes over 40 countries to date with varying access to the infrastructure and funds needed to run a trial that evaluates a multi-domain intervention. The WW-FNFP is open to proposals from the Network that are in need of financial support (up to $1,000,000 (USD) total for up to 3 years). The Alzheimer’s Association recognizes the needs of each study may be varied and the costs to conduct the study may also be varied; we anticipate most requests to be in the $200,000-$400,000 (USD)/year range. The support needed for each study will differ by the infrastructure and resources at each site, and is open to requests that range from start-up costs for infrastructure development, for pilot studies, for supplemental support for ongoing and/or newly established studies or for work that aims to add-on new measures or assessments (i.e. biomarkers) measures.
Funding and award period
The maximum grant amount is up to $1,000,000 (USD) and the project duration is up to 3 years with no minimum timeframe. No indirect costs are allowed on this award. The Alzheimer’s Association, in collaboration with global experts appropriate for the study proposal, will evaluate projects on progress toward specific milestones; continued disbursement of funds is dependent on demonstrated progress toward key milestones.
Eligibility
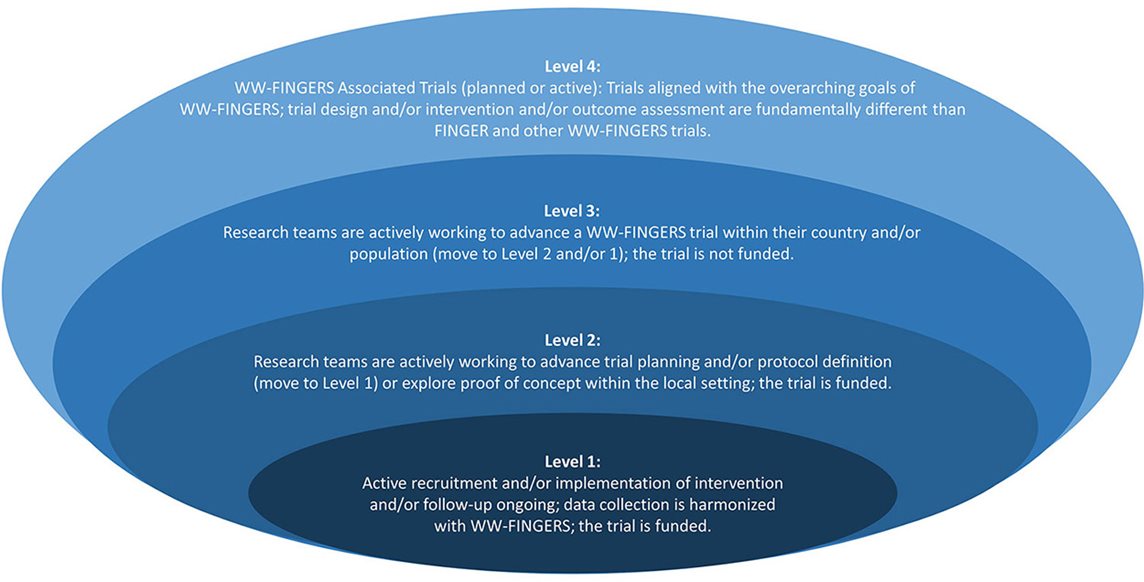
The ALZ WW-FNFP is open to researchers in the WW-FINGERS Network (**To learn more about joining the network, please contact Emily Meyers at easmeyers@alz.org). WW-FINGERS Network studies should have initial discussions with the Alzheimer’s Association team. Studies are eligible only if they are equivalent in planning to a Level 2 or Level 1, as defined in Figure 1 (below) and described in Kivipelto et al (2020) Alzheimer’s & Dementia. Level 3 studies will be considered provided the funding enables them to be moved to a Level 2 or Level 1 study. Level 1 studies that are looking to expand and/or leverage a unique opportunity for their study will be considered.
The Principal Investigator of the project must be a full-time faculty member or full time paid employee of the organization submitting the proposal. Note: Investigators that have received Alzheimer’s Association funding for a related or other study and are currently delinquent in submitting required reports or have awards closed as “Incomplete” are not eligible to apply. For questions about eligibility, please contact the Alzheimer’s Association at grantsapp@alz.org and note that you are seeking clarification regarding the WW-FNFP program eligibility.
Alzheimer’s Association grants are generally open to scientists and researchers across the globe; however, as a U.S.-based charity, the Alzheimer’s Association is subject to, and complies with, U.S. law. As a result, the Alzheimer’s Association cannot award, and will not award, grants in violation of applicable U.S. statutes and regulations. This means, among other things, that the Alzheimer’s Association cannot, and will not, fund any individual or entity (i) that is subject to U.S. comprehensive or targeted sanctions or if awarding funding would result in a violation of such sanctions, (ii) that is on the U.S. List of Specially Designated Nationals or entities owned or controlled by such persons, or (iii) when doing so is otherwise prohibited by U.S. laws related to combating terrorism.
Submission process
Pre-submission inquiries will be accepted at any time. Please use the template provided and include the following:
- Describe the status of the project and what is being requested
- Overview of the geographical location of the study (cultural, economic, sites, etc.), expertise of the study team, information on the population to be engaged and community that will be involved, and a high level description of the proposed intervention.
- Describe future plans or long-term goals of the initiative (i.e. are there opportunities for federal funding, for partnership, other ways for sustainability, etc).
- Level of study as defined by the above criteria and Kivipelto et al (2020) Alzheimer’s & Dementia.
Please send the pre-submission inquiry to grantsapp@alz.org.
Pre-submission inquiries will be welcome at any time; following pre-submission inquiries, applications will be invited for those that meet the program objectives. Proposed deadlines will be discussed with teams invited to submit full applications.
Review process
Pre-submission inquiries will be reviewed by the Alzheimer’s Association with appropriate expertise for evaluation and review on the project plan, feasibility, impact and study team. Following this review, PIs will be contacted and provided additional details for next steps.
Submitting a full application
For those invited to submit a full application, additional materials will be required. Templates and instructions will be provided after approval of the pre-submission inquiry. Full applications will include:
- Executive Summary (1 page).
- Background/ Rationale (1 page): should include background and clearly defined hypothesis and/or rationale addressing why strategy is expected to be fruitful. This should also note the specific aims.
- Work Plan (up to 5 pages): should include goals/specific aims, methods and project plan and should be organized in alignment with the milestones as outlined in the application.
- Project Milestones: workplan should be separated into one-year intervals with specific milestones to be accomplished within that timeframe. Milestones should align with the overall project goals and be designed for easy progress evaluation and for clear determination on whether or not milestones have been met. For each milestone, indicate the relevant Project Aim. (No upload required).
- Principal Investigator(s) and Key Personnel Curriculum Vitae or Biosketch (no more than 5 pages per person); there is no limit on the number of CVs to be included.
- Available Resources and Budget Justification (2 pages):
- Expenses that will not be allowed under this award include: tuition for full degree programs, rent for laboratory/office space, construction or renovation costs, liability insurance, networking and communication fees, and any facilities fees and laboratory/supply costs not directly relevant to the project. If awarded, a full budget of planned expenses will be required.
- Include a list of tools/models available (if appropriate, list critical tools and models to be used or needed in the course of the research). No indirect costs will be allowed. Budget should be broken down in 1-year increments and align with project milestones.
- Citations/References (1 page): Use the reference style that is most common in the major journal(s).
- W9/W8 Documentation.
Additional details
It is the responsibility of the applicant to ensure and verify that:
- The application is submitted by the receipt date/time deadline. Once submitted, you will receive a confirmation email from proposalcentral.com that your application was successfully submitted. If you do not receive a confirmation, click the Proposals tab and under the “Status” column, make sure it says Submitted and not In Progress, which indicates you have not yet submitted your application.
- The application is complete and accurate before submission. Only a single copy of an application will be accepted. We do not require signatures at the time of submission. The signature page provided is for use should your institution/organization require signatures; we do not override any institutional policies and/or procedures. Please do not submit the signature page with your application
- Revisions, additional materials, and/or reference, manuscripts, appendices, etc., are not allowed and, if attached, will be removed from your application. Up to 3 letters of support (no more than 2 pages each) are allowed. Additional letters will be removed
Multiple and overlapping submissions
Multiple submissions from one applicant is not permitted. This includes multiple submissions from the same group and/or collaborators.
Allowable costs under this award
- Small pieces of laboratory equipment and laboratory supplies (purchases over $10,000 require prior approval, even if included in the project proposal budget).
- Computer software if used strictly for data collection and/or analysis.
- Salary for the principal investigator, scientific (including postdoctoral fellows) and technical staff (including modest administrative support).
- Research supplies needed for the proposed studies.
- Support for travel to scientific and professional meetings, not to exceed $1,000 in any given year.
- Open access publications fees are appropriate and reasonable for the project budget. Not allowable as direct costs under this award include:
- Computer hardware or standard software (e.g., Microsoft Office).
- Construction or renovation costs.
- Tuition.
- Rent for laboratory/office space.
- Salary and/or compensation for Alzheimer’s Association staff or for current members of the Alzheimer’s Association Medical and Scientific Advisory Group (MSAG), International Research Grant Program (IRGP) Council.
- No indirect costs are allowed.
Additional information
Ethical/regulatory approvals and reporting requirements
If awarded for funding, the Alzheimer’s Association require that any necessary ethical and/or regulatory approvals are kept current, and may require specific reporting throughout the lifetime of the award. This includes, but is not limited to, the following:
Human participants/ volunteers ethical assurances
Human participants/ volunteers ethical assurances are not required at the time of application. Investigators have up to 90 days after receipt of their award notification to submit these documents. However, the Alzheimer’s Association encourages investigators to initiate their certification applications on a schedule that recognizes that approvals at many institutions can take more than 90 days. We will accept only certifications that apply specifically to the funded project and must include the name of the awardee. An award letter will not be issued unless the appropriate certifications are in place and include the name of the awardee within the 90 days from award notification. The same applies for animal ethical approvals, if applicable.
Annual scientific and financial reports
Interim Scientific and Financial Reports must be submitted at the end of each reporting period as long as the grant remains active. Final Scientific and Financial Reports must be filed within 90 days of the grant‘s end date. All reports must be submitted electronically via proposalcentral.com. The Financial Report must be approved and signed by someone with financial authority in the Office of Research and Sponsored Programs at the recipient‘s institution.
Recruitment efforts for clinical studies
Projects involving human participants must address the appropriate inclusion or exclusion of individuals in the proposed research project and describe recruitment efforts to represent the community in which the study is planned or being conducted. Prior to distribution of funding, the researcher must provide a description of their recruitment plan; including an outline describing how their recruitment efforts will ensure diversity in their participants (visit www.nimhd.nih.gov/about/overview for NIH operationalization). Recruitment efforts should focus on diversity within key target groups, including a diverse representation of, but not limited to: sex, gender identity, sexual orientation, socioeconomic status, race, and ethnicity. This will be tracked throughout the duration of the grant and continued funding is contingent on applications addressing these goals.
Financial responsibility
Funding is awarded to the institution and/or organization, not to the individual principal investigator. The principal investigator or a first-degree relative cannot be listed as the signing official or financial officer, or have checks sent to their attention if awarded.
Appeals of scientific peer review
To maintain a fair and rigorous review system, the Alzheimer’s Association have a process for appeal of funding decisions. Appeals will be not be considered for the letter of intent stage. Regarding applications, an appeal is intended to address extraordinary circumstances.
Appropriate reasons for initiating an appeal might include:
- Evidence that a reviewer has an undeclared conflict of interest.
- An egregious error or misunderstanding in the review process.
- Active malfeasance or demonstrable lack of due diligence.
The appeal process is not intended to provide a mechanism for routine protest of failure to receive a grant.
If an applicant believes an extraordinary circumstance has contributed to failure to receive funding, the principal investigator may send a two-page, double-spaced formal letter of appeal (Word document) to grantsappeals@alz.org. Any supporting documents included must be submitted as a PDF. Appeals must be submitted within 2 weeks from the date your application outcome notification is sent. Notification of action on the appeal will be made via email, usually within 90 days of the appeal deadline.
Nondiscrimination and harassment statement
Alzheimer’s Association is committed to providing an environment free from harassment and discrimination. Alzheimer’s Association strictly prohibits harassment and discrimination based on race; creed; color; religion; sex; sexual orientation; national origin; ancestry; age; Veteran status; citizenship status; marital status; physical or mental disabilities; pregnancy, gender identity or expression (including transgender status); genetic information; and any other characteristic protected by federal, state or local law.